

















The Canadian ARVC registry is a national collaboration of adult and pediatric inherited heart rhythm specialists that will combine enrollment of previously identified ARVC patients (retrospective) with prospective enrollment of newly diagnosed ARVC patients and their families. The focus of the registry will be threefold:
1 ♥
Clinical data collection and evaluation of natural history. This will include novel genetic correlates, evaluation of optimal testing modalities, and identification of risk factors for adverse outcomes.
2 ♥
Genetic testing and gene discovery. Patients' genetic testing results will be collected, and patients that have negative genetic testing will be approached to participate in gene discovery research conducted in Toronto (Sick Kids), Calgary and Newfoundland
3 ♥
Advanced imaging using cardiac MRI. Investigators from the Canadian Advanced Imaging Network (CAIN) will collaborate to determine the optimal utility of cardiac MRI in detection and classification of ARVC
Enrollment in the registry is set to begin in early/mid 2011, with seed funding through the end of 2014. The steering Committee involves Andrew Krahn (Chair, London), Robert Hamilton (Sick Kids Toronto), Brenda Gerull (Calgary), Kathy Hodgkinson (St. John's) and Martin Gardner (Halifax).
Prolonged Monitoring to Detect Ventricular Arrhythmias in Presymptomatic ARVC Patients
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) is a familial condition characterized by onset of life threatening ventricular arrhythmias in early adulthood, presenting with ventricular tachycardia, cardiac arrest or sudden death. The disease is diagnosed with tests that focus on imaging the right ventricle and assessing for ambient arrhythmia or abnormal electrical substrate. These factors are collated into a score that forms the ARVC Task Force Criteria, known to be specific but not sensitive. These criteria have been revised in 2010, introducing a broader and more quantitative approach to diagnosis including genetic testing results, intended to enhance sensitivity without reducing specificity. They account for findings from genetic testing, confounded in part by the unknown significance of a positive genetic test in the absence of a phenotype in a disease with variable penetrance and expressivity. Genetic testing identifies the underlying mutation in 60% of clearly affected patients. Recent access to genetic testing has demonstrated that family members of an affected individual often harbor the culprit mutation, with little evidence that they are affected from clinical testing. Given the risk of life threatening arrhythmia as a first presentation of disease expression, enhanced detection of ventricular arrhythmia would help to identify patients with manifest ARVC.
The PREPARE study will test the hypothesis that prolonged monitoring with an implantable loop recorder (ILR) will provide evidence of progressive electrical disease in gene positive ARVC patients with a non-diagnostic phenotype (negative or mild) who do not receive an implantable cardioverter defibrillator (ICD). Detection of non-sustained ventricular tachycardia will have incremental value over routine periodic clinical follow-up and standard short term monitoring (24-48 hour Holter).

100 gene positive patients without manifest ARVC after standard screening clinical testing will undergo ILR implantation. These patients will fail to meet 2010 revised Task Force Criteria for definite ARVC and will not be considered candidates for a primary prevention ICD by the local investigator. A Health Canada approved St. Jude Medical ConfirmTM loop recorder will be implanted using standard technique with local anesthetic, and patients will be followed for 3 years. Patients will undergo repeat clinical phenotype testing according to the local institutions standard practice, including testing at 3 years after enrollment to reassess Task Force Criteria (standard care), which will constitute the end of the study. A 24-hour Holter monitor will be encouraged annually to provide standard surveillance for ventricular arrhythmia as a comparator to loop recorder findings. In the event that non-sustained or sustained ventricular tachycardia is detected by the loop recorder (=8 beats) and/or Holter monitor, clinical assessment by the local investigator will take place to review the tracing and discuss the findings with the patient. This will follow routine clinical care.
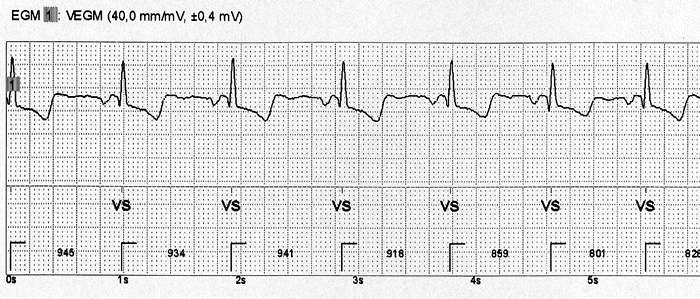
The primary end point is detection of =8 beats of wide QRS complex tachycardia considered ventricular tachycardia by the ILR. Secondary endpoints will include comparison of ventricular arrhythmia burden between routine surveillance Holter monitoring and the ILR, and change in 2010 Task Force Criteria Score from enrollment to 3-year follow-up.
Patients will provide written informed consent to participate in the study, with data collected in a password-protected web based database. Patients will undergo follow-up at 1 and 4 weeks after implant, at 3 and 6 months and every 6 months thereafter. Follow-up will capture findings from loop recorder interrogation, along with change in clinical status and cardioactive drug use. A 24 Hour Holter monitor will be encouraged annually to provide standard surveillance for ventricular arrhythmia as a comparator to loop recorder findings.
This is a pilot study to explore the prevalence and incidence of ambient asymptomatic ventricular arrhythmias in presymptomatic genotype carriers of ARVC. An empiric number of 100 subjects was chosen based on disease prevalence and recruitment goals. End point adjudication will include a 3 member adjudication committee comprised of coinvestigators. A single interim analysis of end points will be performed by an independent Data and Safety Monitoring Committee after 50 patients have completed at least one year of follow-up.